Understanding Certifications for Electronic Hardware Products
Most electronic products require multiple certifications in order to be sold. Which certifications you will need depends on your product specifics and the countries where you will market and sell it.

Don’t overlook the cost and time you will need to obtain all of the certifications necessary for your product.
Certifications may not be the most captivating subject, but it’s essential that you understand all the certifications required for your product.
We’ll mainly discuss certifications necessary in the United States, Canada, and the EU. However, other countries and regions will likely have very similar requirements.
Federal Communications Commission (FCC)
Federal Communications Commission (FCC) certification is required in the United States for all electronic products that oscillate at 9 kHz or higher.
This regulation falls under what the FCC calls “Title 47 CFR Part 15” (15th subsection of the 47th section of the Code of Federal Regulations).
In Europe, there is a similar regulation called CISPR 22. The requirements are very similar, but somewhat stricter in regards to RF emissions at some frequencies. Other countries and regions have similar regulations on electromagnetic emissions.
For all intents and purposes, these regulations include almost all electronic products, since very few products are able to run at frequencies less than 9 kHz.
However, if your product is simple enough you may be able to bypass FCC certification by purposefully designing it to operate below 9 kHz.
For example, some microcontrollers can run at frequencies below 9 kHz. Even then you need to make sure that none of the harmonics exceed the standard limits.
All electronic products with oscillating signals will emit some amount of electromagnetic radiation (i.e. radio waves), generally referred to as EMI, or ElectroMagnetic Interference. Government regulators want to make sure that product emissions don’t interfere with wireless communication.
There are two classes of FCC testing: Class A and Class B. Class A is an easier test to pass, and is intended for products that will be used in industrial applications. Class B is for consumer products and requires stricter testing.
FCC certification can be further split into two types: intentional radiator and non-intentional radiator. The category is determined by whether your product incorporates wireless capabilities such as Bluetooth, Wi-Fi, cellular, or any other type of radio transmitter.
Regardless of the type or classification of your product, if it is AC powered it must also meet conducted emissions limits. This applies whether a product is directly powered from the mains, or whether it is DC powered from an AC adapter that is supplied from the mains.
In general, conducted emissions can be easily suppressed by the use of appropriate ferrite cores. This is the lump often seen close to the DC plugs of AC adapters.
Intentional and Non-intentional Radiators
The FCC classifies an intentional radiator as any product that intentionally transmits radio frequency (RF) waves (also called more broadly electromagnetic radiation). A cellular phone or an Internet of Things (IoT) device are examples of intentional radiators.
A non-intentional radiator is classified as a product that doesn’t intentionally emit radio frequency waves. Every electronic product will unintentionally emit some level of electromagnetic radiation.
Intentional radiator certification is more involved and more expensive than non-intentional certification.
One of the first things you should consider is at what frequency will your product operate? Depending on where your product will be sold, you may not be able to certify some devices that operate at certain frequencies.
For example, some wireless sensor networks operate at sub-GHz frequencies, most likely in the unlicensed Industrial Scientific and Medical (ISM) bands.
In Europe this band is 863MHz to 870MHz, usually referred to as the 868MHz band. In North America, this ISM band is 902 MHz to 928 MHz, usually known as the 915MHz band.
A sensor network operating in the 868 MHz band is not going to get certified in North America, regardless of the radiated power. The same applies to using a 915MHz device in Europe.
One of the best ways to reduce the certification costs for wireless functions in your product is to use pre-certified radio modules. These modules are verified to be within the limits of allowable RF power output levels.
Also, they won’t unintentionally radiate, thus preventing your product from radiating outside of the intended operating frequency band.
One important thing to note is that your choice of antenna can affect certifications. Antenna gain and radiation efficiency can cause the field strength of the radiation to exceed certification limits, even though the output power of the module itself may be within limits.
This is why you should consider using pre-certified modules, with a built-in antenna if possible, for any of your wireless functions.
This will save you the extra cost for intentional radiator certification, since your wireless functions will be performed by the pre-certified modules. Doing this will save you thousands of dollars.
Electromagnetic emissions are measured using a specialized testing chamber called an anechoic chamber (“an-echoic” or non-echoing) which is a specialized room designed to absorb all electromagnetic radiation. The chamber is outfitted with sensors for detecting electromagnetic emissions.
The cost to rent a testing chamber is one of the primary costs of obtaining FCC certification. The rental cost for one of these chambers can be up to $1,000 per hour.
At a minimum, each testing session will take a couple of hours. Most products require several sessions in order to pass.
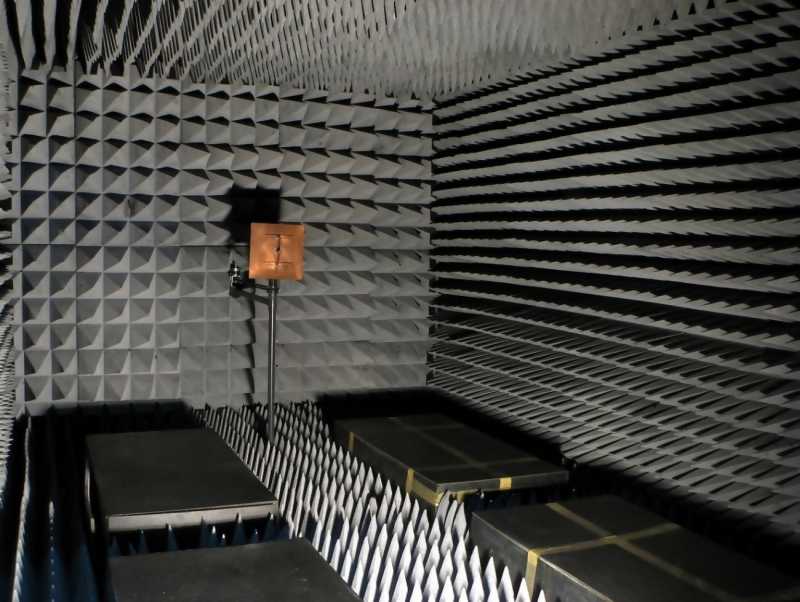
A special anechoic chamber is necessary for measuring electromagnetic emissions.
Most entrepreneurs choose to hire a third party certification testing company such as Intertek or SGS to perform all of the necessary FCC testing.
Typically, you will need to make some modifications to your electronics design in order to pass the emissions testing. This includes such things as adding ferrite beads, capacitors, shields, and other modifications to reduce emissions outside of the intended frequency.
SAR (Specific Absorption Rate)
While EMI is concerned with a product’s interference with other electronic devices, SAR is concerned with the absorption of electromagnetic energy by the human body.
SAR testing is mostly applied to smartphones, tablets and laptops that have high power radio transmitters, and is something to be aware of when designing such products.
UL (Underwriters Laboratories)
UL certification is necessary in the United States and Canada if the product plugs directly into an AC outlet. Primarily the UL is concerned with the electrical safety of your product.
This certification ensures that your product doesn’t start an electrical fire, or cause other safety issues. There are many UL certifications, each tailored to the type of product and its intended use.
For example, one of the most common UL certifications is the UL60950 for information technology equipment. If you have a medical product, you will need UL60601 certification.
Also note that many UL certifications, such as UL60950, will require prior UL certifications that apply to sub-parts of the overall product.
For example, the PCB material in your product along with all the plastic enclosures, should meet the UL94 standard, typically UL94-V0. If your product contains li-ion batteries, these should comply with the UL1642 standard.
Europe generally has its own safety certifications but they are generally very similar to their equivalent UL standards. Usually, a product that is UL-approved needs to have a separate certification to be approved for Europe.
There is a move underway to harmonize these standards. One that is very prominent is IEC62638-1 or its equivalent UL62368. This harmonized standard will supersede UL60950 in North America in late 2020.
Technically, UL certification isn’t absolutely required to sell your product in the U.S. But, if the product does plug into an AC electrical outlet you would be crazy to not get this certification.
If your product starts a fire and you don’t have UL certification, you will be held liable.
Even if no one is ever injured by your product, obtaining a UL certification helps you to make a better, safer product.
Passing these various certifications, whether mandatory or not, helps to make your product more robust and less likely to have any problems in the future.
You don’t want to have issues like Samsung’s Galaxy Note 7 phone that was constantly catching on fire. Regardless of the size of your company, recovering from these types of failures can be next to impossible.
UL certification is only necessary for products that plug into an AC power outlet. Most battery powered products need to have their battery recharged at some point with an AC power outlet.
You can avoid this UL certification requirement if your product uses a pre-certified stand-alone charger. One caveat to mention is that this applies to SELV (Safety Extra Low Voltage) circuits.
In a nutshell, that means no operator accessible parts, including the output of the AC adapter, should have a voltage higher than 42.4 VAC peak (30VAC RMS), or 60 VDC.
So, for example, if your product can be recharged by a USB charger, then the UL requirement falls on the charger itself and not necessarily on your product.
In this case you could either purchase a pre-certified USB charger to bundle with your product, or you could require the customer to supply their own USB charging source.
The same is true if your product uses a non-USB charger such as a wall adapter power supply. In this case, once again the UL certification requirement falls on the wall adapter since it plugs directly into the AC electrical outlet.
Your product will never see that AC voltage since the wall adapter converts it down to a low DC voltage.
Most product liability insurance companies, as well as most large retail chains, will require that your product be UL certified even if it doesn’t plug directly into an AC outlet. Larger retailers will require it as an extra margin of safety.
This is one reason that many entrepreneurs begin selling their product directly to consumers via their own website. Doing so may allow you to minimize the number of certifications required.
UL certification can be quite complex and confusing because of the numerous types of UL certifications.
If your product does plug directly into an AC electrical outlet then I highly suggest you consult with an expert on UL certification to review the design before you proceed too far with development.
CSA (Canadian Standards Association)
CSA (Canadian Standards Association) is an alternative to UL certification and is valid in both Canada and the United States.
CE (Conformité Européenne) Marking
CE marking is required for the majority of products marketed in Europe.
CE is an abbreviation for the French phrase Conformité Européenne which translates to European Conformity. Originally called an EC Mark, this certification officially became known as a CE Marking in 1993.
The CE marking on a product is a manufacturer’s declaration that the product complies with the health, safety and environmental requirements in Europe. It is quite similar to a combination of the UL and FCC certifications.
CEC (California Energy Commission)
If your product contains a battery charger, and will be sold in the state of California, then it must meet the California Energy Commission (CEC) efficiency requirements.
In particular, the AC Adapter must be certified to the DOE (Department of Energy) energy efficiency level VI.
RoHS certification
RoHS certification verifies that a product contains no lead, or other harmful substances such as Cadmium and Mercury. RoHS is necessary for products sold in the European Union and the state of California.
Since most products are sold in California and/or Europe, their requirements have become the de-facto standard for environmental regulation.
RoHS is one of the easiest and cheapest types of certifications to obtain. In fact, you may find this is something your contract manufacturer will do for you.
Just make sure that the manufacturer is following the latest amendment of the RoHS 2 standard. Please also note that RoHS encompasses everything in your product, including the screws and enclosure.
Also remember, you don’t want to mix leaded and lead-free components on the same PCB assembly as the reflow profile for each type are incompatible.
That’s why I recommend that from the very beginning you select and work with lead-free components.
Most components are available in leaded and lead-free versions. If you select leaded versions for prototyping and development you will have to go through the time consuming exercise of reworking your Bill of Material for the manufactured product to be RoHS compliant.
WEEE (Waste Electrical & Electronic Equipment)
The Waste Electrical & Electronic Equipment (WEEE) regulation is a directive in the European Union that designates safe and responsible collection, recycling and recovery procedures for all types of electronic waste.
WEEE encourages the design of electronic products with environmentally safe recycling and recovery in mind.
This regulation works in conjunction with RoHS. RoHS regulates the hazardous materials used in electronic products, and WEEE regulates the safe disposal of the product.
ESD immunity
ElectroStatic Discharge, or ESD, is easily produced and can damage your product. For example, the triboelectric effect of simply walking on a carpet can produce enough static charge to damage sensitive electronic equipment.
While generally not a safety issue, ESD immunity testing is highly recommended. While some ESD induced failures are immediate, others may not manifest for a while.
ESD immunity testing prevents you from encountering problems some time down the road, when a lot of products are already in the field. The cost of finding out that your products are failing at a high rate, especially after the revenue from the sales has been divested, can be devastating.
The accepted test protocol for ESD immunity is the IEC 61000-4-2, with at least Level 2 passing. However, I recommend that you aim for Level 3 or higher.
ESD is usually mitigated through the use of transient voltage suppressors (TVS) on exposed pins, such as USB, of the product. I recommend that you allow some space on the PCB for such components, even if they might prove unnecessary in final product.
Bluetooth SIG
Although not technically a certification, if your product incorporates Bluetooth Classic or Bluetooth Low-Energy, then you must pay a licensing fee and testing costs depending on your product implementation.
Bluetooth SIG is a non-profit organization that oversees the Bluetooth standard and the licensing of the Bluetooth technology trademark. You must pay them a fee to use the Bluetooth trademark.
If your product implements Bluetooth using a pre-certified module, then you only need to pay the licensing fee.
However, if your product incorporates a custom designed, non-certified Bluetooth radio then you will also need to pay for additional testing and certification.
The Bluetooth SIG licensing fee alone is $8,000 USD. They used to also offer a lower cost option specifically for startups, that costs only $2,500 USD. However, in early 2020 they eliminated this lower cost option.
One way to eliminate the need for this licensing fee is to avoid using the trademarked word “Bluetooth” on your packaging and marketing materials.
Unlike most other certifications which are regional, any Bluetooth licensing fees or testing costs will apply at an international level.
Other Certifications
Some types of products will require even more certifications. For example, toys have a very comprehensive list of required tests and regulations to ensure they are safe for children.
Or, if your product comes into contact with food then you’ll need to follow FDA guidelines on what materials can be safely used.
Because lithium batteries have the potential to cause a fire hazard (think Samsung Galaxy Note 7), there are regulations on the shipment of lithium batteries.
The air shipment of bulk lithium batteries is especially restricted to cargo aircrafts only. Also, if your product contains a large li-ion battery, there are further restrictions based on its Equivalent Lithium Content (ELC).
Final Thoughts
You don’t want to certify your product too early, because your product will have to be completely retested if any design changes are made.
However, you should plan for certification even during the design phase. For example, make sure to select lead-free components from the start since your final design will be required to meet the RoHS standard.
Testing is expensive and takes up to a month to complete (depending on the testing facility’s queue), so you don’t want to do it more times than is necessary. Wait until you have manufacturing, and most bugs, figured out.
Then, submit a production unit for certification testing. Just be sure to plan for the time that testing and certification will take. You won’t be able to ship your product to customers until the certification is completed.
Regulations often require a copy of your instructions manual to be included along with the units to be tested. So be sure to have your manual finalized before you begin certifications testing.
Regardless of your product, or the countries where it will be sold, you would be very wise to hire someone that is an expert in all the various required certifications.
For example, we have several experts in certifications inside the Hardware Academy. In fact, you get access to an entire team of various experts to help bring your product to market.
It is extremely unlikely that your design engineer(s) will have the necessary knowledge to ensure your product smoothly passes all of the various certifications required.
Many startups plan to market their product globally without understanding that they need capital to pay for added regulatory tests for each country.
I highly recommend that you focus your initial efforts on a single country or region, then expand slowly from there.
The USA and Canada share similar certification requirements so in most cases you can sell your product in both countries with a single set of certifications. The EU has the advantage that one set of certification requirements are valid for multiple countries.
Asia on the other hand tends to have separate regulations for each country. Unless you live in Asia, marketing a product there will only be a viable option once you are a significantly sized company with people on the ground there.
Although you don’t want to actually begin the certification process until you have a production-quality unit for testing, it’s still a good idea to understand all the various issues surrounding certifications.
Inside the Hardware Academy we have a very in-depth training course that covers all of the details on how to certify a new product for the United States, Canada, and Europe.
Other content you may like:
- Certifications Required to Sell a New Electronic Product [YouTube]
- Introduction to Power Sources for Modern Electronic Products
- From Prototype to Mass Manufacturing: Understanding Scaling Costs for Physical Products
- How to Design a Wearable Technology Product
- Lesson 4: The Strategic Way to Develop and Sell Your New Electronic Hardware Product






So if our AC power supply supplier (we include a 110-240V to 12V AC adapter with our product) is CE certified and SAA, do we also need UL certificate to be selling in the USA? Please your help!
Thank you John!
Yes, it would need to also have UL certification for selling in the USA.
Hi John, for Wi-Fi Alliance, is it the same case as BT “eliminate the need for this licensing fee is to avoid using their logo or trademark? Thank you.
If a product bears the CE symbol, it means that the maker has evaluated it and determined that it complies with safety, health, and environmental protection standards.It is necessary for all goods produced in the globe that are later commercialised.CE MARK Certification in indonesia A legislative requirement known as Conformite Europeenne certification attests to a product’s safety for use and sale in the European Economic Area.On certified products, manufacturers affix the CE label to show that the item complies with European safety regulations and is permitted to be traded freely within the EEA.are used by manufacturers to show that a product complies with rules and directives regarding environmental, health, and safety concerns.The market is open to products bearing this marking.As a result, the CE marking can be compared to a trade passport because without sufficient proof of noncompliance, member states cannot restrict the placement of items with the CE on the market.
Thanks for sharing this here Sindhu!
Hi John, Thanks for the information. For CEC, if our product has only a USB-C cable and not any wall-adapter does it need to be compliant to CEC? If so, how would it be tested?
What about software specifically? What are standards covering consumer electronics explicitly? I’m familiar with IEC 61508, and DO-178C, but is there an equivalent for consumer electronics? Is any software standard required?
Hi! Great article. Quick question. Is there a requirement to put the various certification symbols (like FCC, CE, ROHS, country of origin, etc etc) on the actual device, or can it be only on the packaging the device ships in? I’m making a small wearable. Technically I could put a label on it with these marks, but it would be very ugly and cheapening. Can I avoid putting labels on it? Thanks!
Hi John,
Thanks for the rich content, yet I was still wondering what is the link between those certifications and IPC standards?
Thanks.
Hi John,
Thank you for the awesome information! Do you have any advice on how a product with Bluetooth can be commercially viable with the Bluetooth SIG licencing fees? We have a prototype for a product and would be close to manufacturing if not our calculations that show that the amount of money we have to pay Bluetooth SIG even before we can validate that customers are ready to buy it, makes everything too expensive. Unfortunately we wanted to use Bluetooth in the product as a differentiator, so we cannot avoid it.
Thank you!
Thanks David. The easiest workaround is to simply not use the word “Bluetooth” on any of your marketing materials and instead use more generic terminology like “wireless communication”. I know you mentioned BT being a differentiator but you could still have it but just without using the brand name. For your initial sales tests you may even find a way to manually tell customers that it has Bluetooth without having it printed on any marketing materials.
Thank you for your advice!
Hi John,
Relating to this query, I would like to clarify one thing, ” We can use a pre-certified Bluetooth/BLE module in our product and we don’t have to pay any license fees if we don’t use the term “Bluetooth” or “BLE” in any of the printed document regarding the product”. Did I understand you correctly??
Thanks,
Nithin
Correct, the licensing fee is for allowing you to use the trademark Bluetooth. If you never state your product uses Bluetooth then you don’t need to pay the licensing fee.
Hi John,
Thank you very much for your reply. This article and your support are helping hobbyists like me to develop our own real products.
Thanks again,
Nithin.
A few years ago, I invented, patented, and licensed a counterfeit bill detector device. We are in the process of providing a cell phone type power bank to power the device and I have a question about certifications.
We have found a couple of suppliers for the power bank wall charger. One supplier has CE-EMC and RoHS certifications on their wall chargers. The other has ETL and FCC certifications. Is the CE-EMC or ETL certification an acceptable replacement for the UL certification or should we only use a UL certified wall charger? Also, are there any other important necessary certifications we should have for the wall charger like RoHS, CEC, SA, Weee, or ESC ?
Thanks and great, useful article,
Robert – Las Vegas
Hi Robert,
If the product is to be used in NA, then you will need the ETL/FCC certified product.
Additionally, for CA, you will need to have the California RoHS. Typically though, if you are RoHS3 compliant, you also meet the CA RoHS requirements.
Finally, your product must meet the latest EPA/DoE Energy Star Level VI efficiency requirements.
Hope that helps.
Shawn – Contributor on the Hardware Academy
Would UL/FCC be better for NA than ETL/FCC? I can get either for the same cost. Also, do we need CEC to sell in California?
What is the difference between FCC 47CFR 18 and FCC Class A or B certification?
Hello, The readers may get confused because of your references to the UL and CSA mark.The MARK should not be associated to the standards.These a MARK as wkk as ETL, Nemko or MET. The products may be compliant to the standards, not the mark.
Hi John,
That’s very helpful! I have a question, our lab developed an electronic system, and we would like to sell it to several collaborators (other labs) in our field to get some feedback in order to improve the system, in this case, do we still a certification, like FCC?
Thank you!
Hi Leo, thanks for the comment. The answer is it really depends. If you are considering this to be early sales tests and the product is not classified as an intentional radiator then it likely will not need FCC certification.
I’ll be happy to discuss this with you in much more detail if you wish inside my Hardware Academy platform.
That’s a great thing I’ve learned, thanks for learning us.
May I ask what is the difference between the UL / CSA certification compare with the CE marking?
Hi do you know of a chart that shows the different electrical certifications needed by country – UL, CE, etc? Thanks!
Very informative article John. Thank you.
I have some questions that I hope you can help me with:
1) Does a USB charging plug (the ones that just plug into a wall outlet and has the USB slot on the other end) require FCC certification?
2) Does the USB charging plug require any UL certification? If so what is the UL standard no?
3) Is the USB charging plug required to meet CEC efficiency requirement?
I appreciate for any advice/help.
Thank you,
Fuji
Thanks…. for such information
I’d like to sell a flying insect exterminator small machine in the US. The manufacturer is in Italy, the machine have a certificate of CE93 E.M. C, Do you think I need the UL for USA? Can you please let me know which certification do I need to certify this product for USA.
Thanks
Oscar
Hi John,
Great overview and intro to the exciting topic of certifications! 🙂
I am building a smart power strip with a weird enclosure design. Most of the internal electronics are off-the-shelf, though I am not 100% sure on all the certifications of all the components. Let’s for now assume the components have the necessary certifications in isolation (before getting integrated into my product).
But I am struggling figuring out which certifications I need to sell the overall product into the North American market. I am particularly stuck on UL, where there are lots of different standards that might apply (1363, 1449, 60730, …). I am even more confused what it even means now to be “UL certified” given that there are so many standards that could apply.
Who exactly can determine which standards apply? I don’t even know who to go to and how reliable their advice is. Could I just go to an accredited lab and just say, “hey make this thing UL certified” and they will come back with the standards/categories and a price estimate ?
Thanks for your guidance!
Hey Ronald, thanks so much for the comment and great question!
Since you are a member of the Hardware Academy would you mind reposting this question in the Academy community area? That’s a much better place to get answers to these types of questions.
Thanks!!
I’d like to sell tattoo machines in the US. The manufacturer is in China.
Which certification do I need and where/how do I start going through this process?
Thanks
Hey Roman, You can also try to use dropshipping with the help of shopify.
That can help you easily sell tattoo machines from China to USA.
Hi John,
I have a delicate question to FCC certification. You say that
Question:
Is a class-A audio-amplifier an oscillating device, if there is no oscillator? At least it doesn’t oscillate by itself.
Case ‘yes’:
then a class-A audio-amplifier for bass-frequencies up to 9 kHz doesn’t need FCC?
What about distortion from clipping?
What about class-A/B, B, C, D?
I highly appreciate your help!
With regards,
Hans
Hi Hans,
No, an audio amplifier alone will not require any FCC certification.
However, a class D audio amplifier can generate harmonics well into the RF range, so some in-house EMF testing is usually a good idea for a class D amp.
Hope this helps!
John